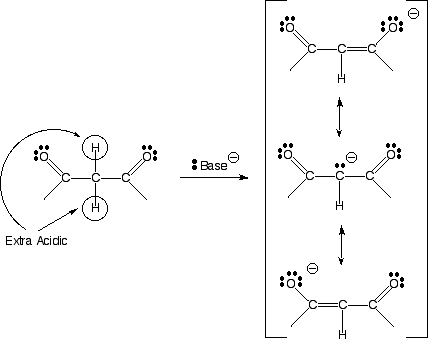
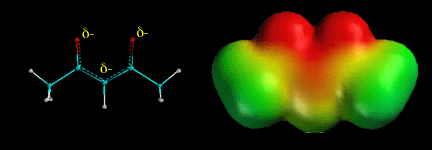
The C-H hydrogen atoms between two carbonyl groups are aven more acidic than normal a hydrogens because the resulting anion is double resonance stabilized. The above electrostatic potential surface shows how the negative charge (red color) is spread over all three atoms as predicted by the three resonance contributing structures.